Abstract
Purpose: A few patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL) who start on standard primary treatment with R-CHOP do not complete the recommended 6-8 cycles for reasons related to toxicity, however, this group has seldom been formally studied. We set out to describe the outcome of DLBCL patients failing to complete at least 6 R-CHOP cycles for reasons other than non-response, and to investigate determinants of such failure.
Methods: We identified patients diagnosed with DLBCL in Sweden 2007-2014 who started primary treatment with R-CHOP (i.e., received at least one cycle) with the aim to receive 6 or 8 cycles, in the national Swedish Lymphoma Register (N=2,155). The analysis covered 5 out of 6 health care regions (70% of all patients diagnosed nationally). Overall survival by number of cycles was estimated using Kaplan-Meier curves (with censoring at stable disease or progression at first interim evaluation to avoid inclusion of patients who switched therapy due to non-response). Univariable and multivariable logistic regression was used to estimate odds ratios (OR) with 95% confidence intervals (CI) for the association between baseline clinical and demographic characteristics and failure to complete full R-CHOP treatment (i.e., receipt of less than 6 cycles compared with 6 cycles or more). Baseline characteristics included age, sex, calendar period of diagnosis, performance status, Ann Arbor stage, serum-lactate dehydrogenase, hemoglobin level, number of extranodal disease locations, and international prognostic index (IPI) score. In the logistic regression analysis, patients with early tumor progression (stable or progressive disease at interim or final evaluation) were excluded.
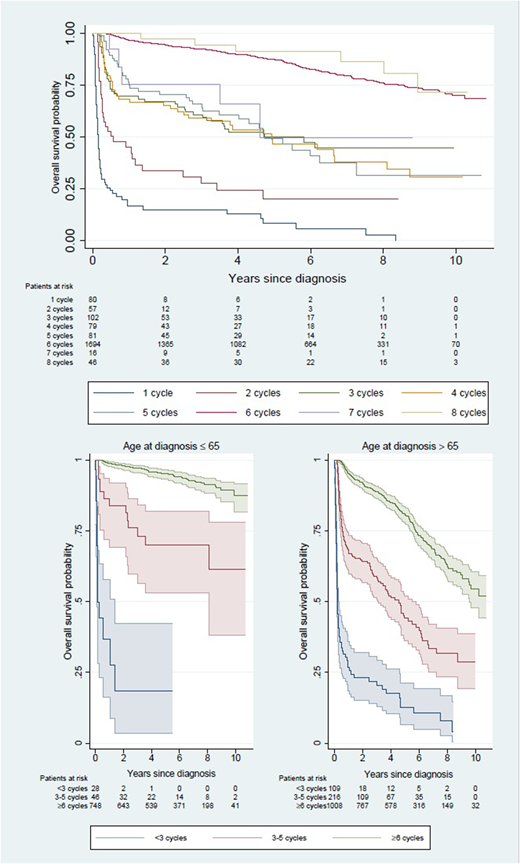
Results: Among the 2,155 patients who started on R-CHOP, 399 (18.5%) failed to complete 6 cycles. Of these, 137 patients (6.4%) received only 1 or 2 cycles, and 262 (12.2%) received between 3 and 5 cycles. Only 42 patients stopped prematurely due to evidence of non-response whereas the majority (357 out of 399 patients, 89%) did so for other reasons related to toxicity and early death. Patients who failed to complete 6 cycles were older than other patients (median age 77 versus 68 years), more often had a WHO performance status of 2 or more (33% versus 12%) and more often were diagnosed with stage IV disease (40% versus 33%). Number of received R-CHOP cycles strongly correlated with survival (Figure 1). Among patients 65 years of age or younger, 5-year OS varied from 18% (95% CI 4-42%) following <3 cycles, to 70% (95% CI 53-82%) following 3-5 cycles, and 95% (95% CI 93-97%) following at least 6 cycles. Among patients older than 65 years, the corresponding figures were 13% (7-21%), 44% (95% CI 36-52%) and 81% (95% CI 78-84%), respectively (Figure 1). Using logistic regression, old age, poor performance status, hemoglobin levels <100, >1 extranodal disease location and high IPI were statistically significantly associated with failure to complete at least 6 R-CHOP in univariable analysis. In a multivariable model, old age, poor performance status and >1 extranodal location remained significantly associated with failure to complete 6 cycles of R-CHOP for reasons other than non-response.
Conclusion: A surprisingly large proportion of patients diagnosed with DLBCL intended for treatment with 6-8 R-CHOP cycles fail to complete the treatment at the population-based level for reasons unrelated to non-response. Failure to complete at least 6 R-CHOP cycles was associated with poor survival, especially for patients receiving 1-2 cycles only, but also for those receiving 3-5 cycles. Old age and poor performance status most strongly predicted such failure.
Figure 1: Kaplan-Meier curve of overall survival of patients with DLBCL diagnosed in Sweden 2007-2014 by number of administered R-CHOP cycles overall (top panel) and by age at diagnosis above or below 65 years (bottom panels).
Ekstrom Smedby:Janssen Pharmaceuticals: Other: The Department have recieved partial funding from Janssen Pharmaceuticals. Harrysson:Janssen Pharmaceuticals: Other: The Department have recieved partial funding from Janssen Pharmaceuticals. Ekberg:Janssen Pharmaceuticals: Other: The department has received partial funding from Janssen Pharmaceuticals. Eloranta:Janssen Pharmaceuticals: Other: S Eloranta is currently employed as a project coordinator and her salary is funded via a public-private real world evidence collaboration between Karolinska Institutet and Janssen Pharmaceuticals.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal